4th Jun 2024
Should Pharmacies Use Cold Storage Monitoring?
There are numerous rules and regulations in place to ensure that consumers trust the items they consume. From food to makeup to medicine, the steps taken to guarantee satisfaction are extensive. The pharmaceutical industry is one of the most important sectors that requires precise following a wide range of rules.
Pharmaceutical products are sensitive to temperature variations. Deviations from the recommended storage conditions can render medications ineffective or even harmful.
This begs the question: should pharmacies use cold storage monitoring systems to safeguard these valuable products? Continue reading to gain a comprehensive understanding of the subject.
Two Types of Cold Storage: Refrigerated and Frozen
Refrigerated Cold Storage for Pharmacies
Refrigerated storage—ranging from 2°C to 8°C (35°F to 46°F)—is essential for preserving many pharmaceutical products. This temperature range is crucial for biologicals, vaccines, and certain medications that are sensitive to heat or light exposure.
By maintaining the integrity of active pharmaceutical ingredients (APIs), refrigerated storage ensures that medications retain their therapeutic properties until they reach the patient. Temperature excursions outside the recommended range can lead to the degradation of APIs, reducing the drug’s effectiveness and potentially leading to harmful consequences for patients.
Frozen Cold Storage for Pharmacies
Frozen storage demands maintaining temperatures at or below -20°C (-4°F). This storage method is critical for certain high-value pharmaceuticals, including some biologics and advanced therapeutic products such as cell and gene therapies. These products are often highly sensitive to even slight temperature increases. Thus, they require stringent control to prevent any denaturation or loss of potency.
Effective frozen storage infrastructure encompasses precise temperature monitoring systems and backup solutions to guard against power failures or equipment malfunctions. These systems ensure that the pharmaceutical products remain stable and safe for use.
Rules and Regulations of Cold Storage
Several organizations that oversee the safety, efficacy, and quality of pharmaceutical products set the rules and regulations governing pharmaceutical cold storage. Below, we’ll discuss the key organizations that establish guidelines for pharmaceutical cold storage and enforce compliance.
Food and Drug Administration (FDA)
The US Food and Drug Administration (FDA) is a regulatory agency responsible for protecting public health by ensuring the safety and effectiveness of pharmaceutical products. It establishes rules and regulations for pharmaceutical cold storage to safeguard the quality and integrity of temperature-sensitive medications.
World Health Organization (WHO)
The WHO sets global standards for the storage and distribution of pharmaceutical products, including those that require cold storage. The WHO’s guidelines help ensure the quality and integrity of medications worldwide, particularly in resource-limited settings.
United States Pharmacopeia (USP)
The United States Pharmacopeia (USP) is a nonprofit organization that sets quality standards for medications, dietary supplements, and healthcare products in the United States. Pharmacies and healthcare facilities rely on USP standards for cold storage to meet regulatory requirements, maintain product quality, and uphold patient safety.
Specifically, the USP outlines guidelines for pharmaceutical cold storage to maintain the stability and integrity of temperature-sensitive medications. These standards detail the recommended storage conditions, including temperature ranges, humidity levels, and storage duration.
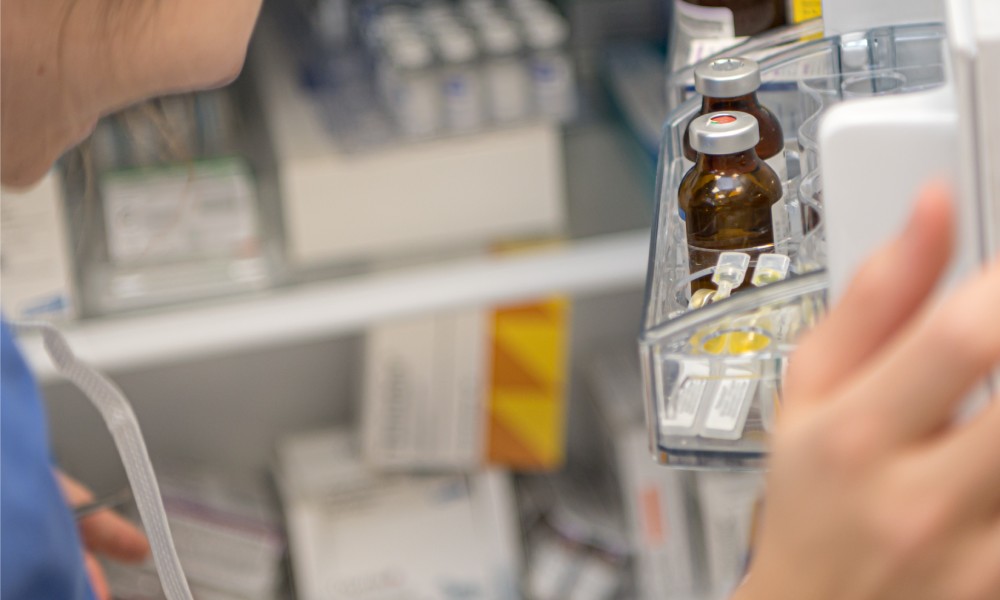
Items That Demand Cold Storage
Proper cold storage monitoring is essential for maintaining the efficacy and safety of numerous pharmaceutical products. Certain medications, vaccines, and biological samples must remain within specific temperature ranges to prevent degradation.
Vaccines
Vaccines are highly sensitive biological products that must stay at specific temperatures to retain their potency. Exposure to temperatures outside the recommended range can lead to vaccine degradation and reduced efficacy.
Biological Drugs
Biologics, which include proteins, antibodies, and other complex molecules, are prone to denaturation and degradation if not stored at the proper temperature. Cold storage is essential to preserve the integrity of these sensitive drugs.
Insulin
Insulin, a hormone crucial for managing diabetes, must remain refrigerated to prevent degradation. Fluctuations in temperature can impair the insulin’s effectiveness, potentially endangering the patient’s health.
Some Antibiotics
Certain antibiotics, especially those in liquid form, may require refrigeration to maintain their stability. Failure to store these antibiotics at the appropriate temperature can result in reduced efficacy or even bacterial resistance.
Temperature-Sensitive Medications
Beyond vaccines and biologics, various temperature-sensitive medications need cold storage to prevent chemical breakdown. These medications may include certain cancer drugs, hormone therapies, and specialized formulations.
Diagnostic Reagents
Reagents used for diagnostic tests, such as enzyme-linked immunosorbent assays (ELISAs), often require refrigeration to remain viable. Proper storage of these reagents ensures accurate test results and reliable diagnostics.
Blood Products
Blood products such as red blood cells, platelets, and plasma derivatives need to remain at controlled temperatures to maintain their viability and prevent spoilage. Cold storage is essential for ensuring the safety and efficacy of these critical products.
Some Oral Suspensions
Certain liquid medications, particularly oral suspensions that users must reconstitute before administration, may require refrigeration to prolong their shelf life and maintain their therapeutic properties.
How Can Pharmacies Regulate Temperatures for Cold Storage?
The implementation of cold storage solutions in pharmacies necessitates robust monitoring systems to maintain these specific temperature ranges.
Backup Power Solutions
Power outages are a nightmare. If cold storage machines lose power, the medicinal products within become unreliable and unsafe.
Pharmacies mitigate this risk by installing backup power solutions, such as uninterruptible power supplies (UPS) and generators. UPS systems provide immediate, short-term power during outages, ensuring refrigeration units remain operational until generators take over.
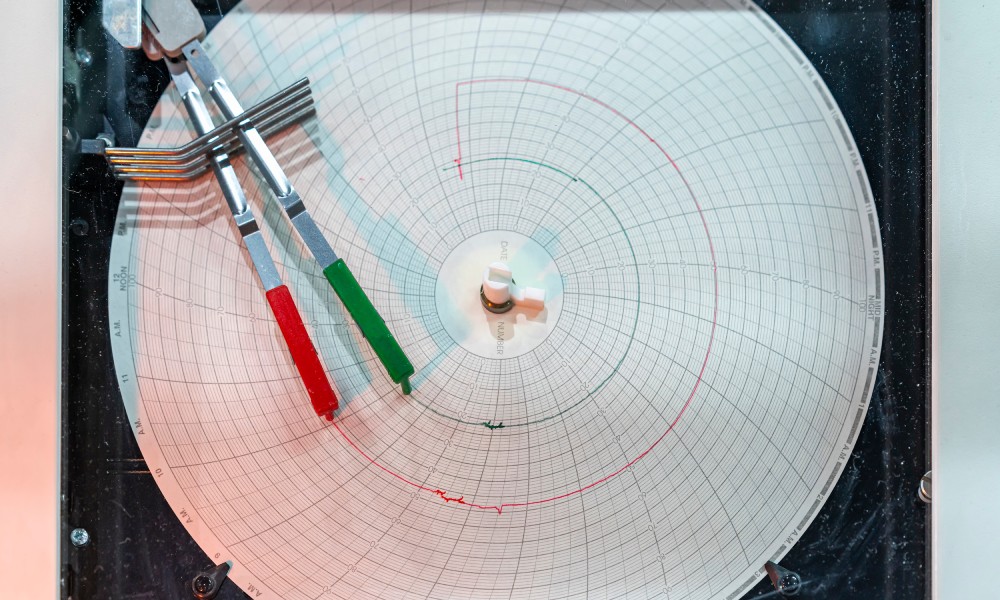
Chart Recorders
Chart recorders offer a traditional yet reliable method for tracking temperature fluctuations over time. These devices use a pen or needle to plot temperature data onto circular chart recorder paper.
The primary advantages of chart recorders are their durability and simplicity. Unlike digital systems that may require frequent calibration or are susceptible to software issues, chart recorders function autonomously without the need for an internet connection. The paper charts can serve as a tangible archive for auditing purposes, compliance with regulatory requirements, and verifying the historical integrity of temperature-sensitive pharmaceutical products.
Standard Operating Procedures (SOPs)
Standard Operating Procedures (SOPs) are detailed, written instructions designed to ensure the consistent execution of various processes within a pharmacy, including cold storage monitoring. SOPs provide a step-by-step guide that outlines the responsibilities, actions, and protocols to be followed by pharmacy staff to maintain the integrity of temperature-sensitive pharmaceuticals.
Furthermore, SOPs serve to minimize human error by standardizing procedures. With clearly outlined instructions, staff are less likely to deviate from established practices.
There’s no need to ponder if pharmacies should use cold storage monitoring; the answer is clear. Pharmacies must use cold storage to guarantee the potency and security of every medicinal product.
Let Recorders Charts and Pens be a part of ensuring the pharmaceutical industry is safe and operational. With various chart recorders and replacement parts, we have the resources you require.

